The role of mechanosensing and antibodies for interactions of Plasmodium sporozoites with neutrophils in 3D environments
The new project started in
3rd funding period (July 1st, 2022)
Project description – Summary
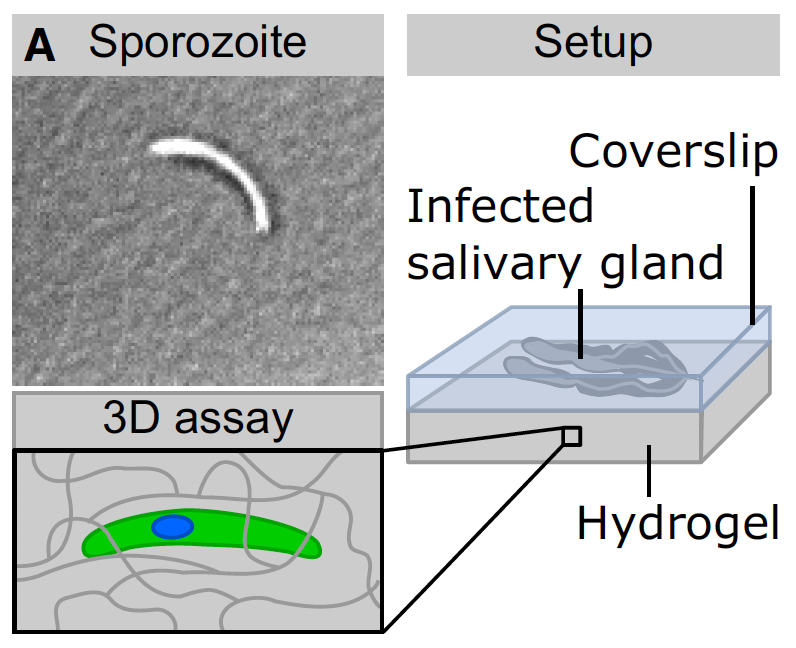
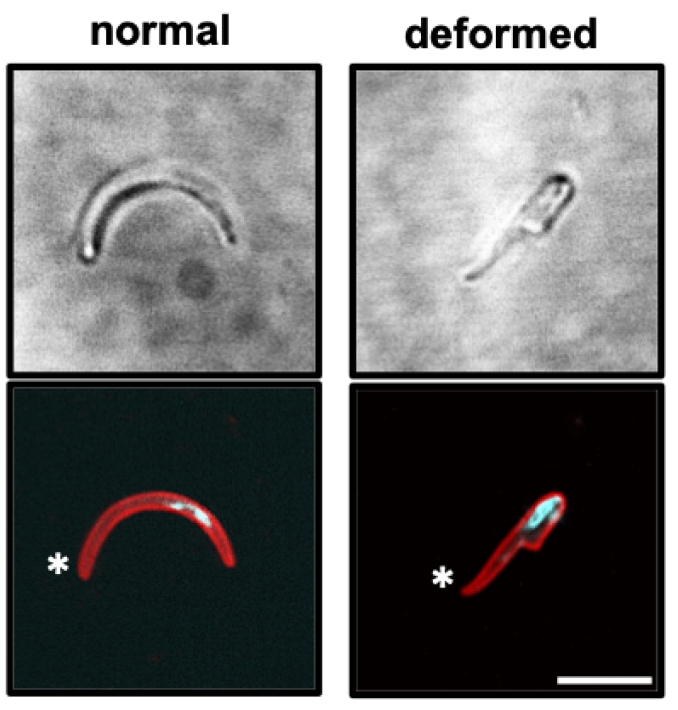
At the beginning of a malaria infection, a mosquito injects Plasmodium sporozoites into the host skin, where they migrate at high speed through the dermis in search for a blood vessel to continue to the liver. For migration, sporozoites use gliding motility, which is much faster than the crawling of mammalian cells. Surface proteins of gliding parasites represent a target for antibodies, the binding of which can stop motility and hence prevent a malaria infection. However, it is not clear how the sporozoite achieves efficient gliding, adapts to different environments and how antibodies immobilize it. Sporozoite migration relies on different proteins on the parasite surface, but their interactions with the environment are unclear. A key protein for sporozoite migration is TRAP (Thrombospondin-related anonymous protein). Deletion of this protein prevents entry of sporozoites into the mosquito salivary glands and hence sporozoites are not transmitted. TRAP contains two adhesive domains of which one is an ancient I domain of integrins. We recently suggested that this adhesive I domain of TRAP might have evolved to bind promiscuously to many ligands. We found that mutations in the I domain that change the overall surface charge allowed sporozoites to colonize the salivary gland but the parasites were dramatically reduced in their capacity to migrate on glass and to infect rodents. How these mutants migrate in the skin is not clear. An additional key factor in migration seems to be the elasticity of the surrounding tissue, with sporozoites moving on hard fibroblasts but not on soft endothelial cells. Within the skin, the immune system targets sporozoites. Antibodies bind to the major surface protein of sporozoites, CSP (circumsporozoite protein), which can lead to sporozoite death. Antibodies can also immobilize sporozoites and opsonize them for phagocytes such as neutrophils. Anti-CSP antibodies are currently being investigated as preventive tools and the only approved malaria vaccine is based on CSP. Yet, how these antibodies stop sporozoite motility is not clear. We recently found that the skin provides a challenging mechanical barrier for sporozoites as sporozoites lacking the protein concavin disintegrate during migration. To investigate the question of how sporozoites migrate through the skin in a realistic situation, skin-mimetic materials with defined 3D structures and elasticity are required. We therefore aim at pushing the limits of current 3D structuring methods so that materials that resemble the structural and mechanical properties of skin are generated and applied to the open questions of sporozoite migration and its inhibition by antibodies. To analyze parasite spread in the human host, we aim to develop skin mimetics of increasing complexity.

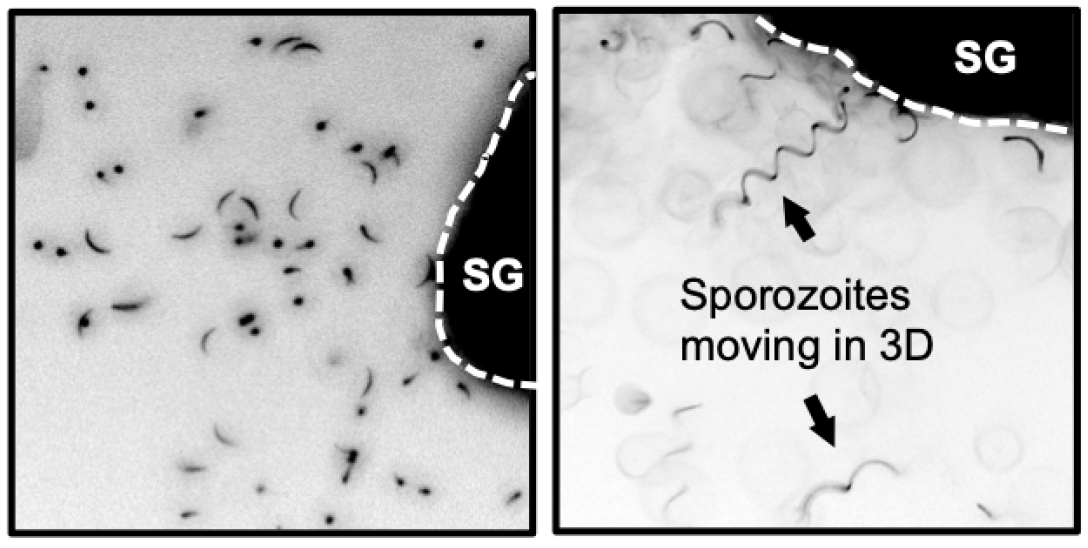
The key aims of our project are (i) to understand sporozoite adhesion and migration through generation of tunable 2D substrates and a 3D skin-mimetic lab-on-a-chip system and (ii) to decipher interactions between sporozoites and neutrophils for elucidating killing mechanisms in the presence and absence of antibodies.
To achieve these goals, we will (i) examine migration of wild type and mutant sporozoites on a range of substrates and cell lines with distinct ligands and elasticity, (ii) establish a tunable skin-mimetic system that allows the variation of pore-size, substrate ligands and elasticity, (iii) perform in vitro and in vivo assays to determine sporozoite-neutrophil interactions and their modulation by anti-sporozoite antibodies.
Project Staff


