Dynamics of events in HIV-1 replication
Project description – Summary – 3rd funding period
In this project, we want to elucidate the dynamic events in the early phase of HIV-1 replication, from entry of the viral capsid into the cell up to release of the viral genome („uncoating“) for integration into the host cell DNA. Towards this aim, we develop and apply fluorescent labeling strategies that allow us to visualize entering viral complexes by live cell imaging and super-resolution microscopy.
In order to follow the capsid on its journey through the infected cell, we have developed a minimally invasive strategy to tag the capsid protein CA with fluorescent dye molecules, based on genetic code expansion (GCE) and click-labelling (Schifferdecker et al.). We have also collaborated with the Kräusslich group to establish detection of newly synthesized HIV-1 DNA by live cell microscopy (T. Müller et al., Elife 2021).
Using these tools, together with correlative light and electron microscopy (CLEM) conducted by Kräusslich and Lusic/Beck in collaboration with us (Zila, Margiotta et al., Cell 2021) lead to a revised model of HIV-1 post-entry. While it was initially assumed that the role of the capsid is mainly to deliver the viral genome into the cytosol, results from us and others indicate that CA stays associated with the viral genome up to the nucleus and even during passage through the nuclear pore. Completion of reverse transcription of the genome and its release from the capsid apparently occur in the nucleus, when the HIV-1 has reached the site of integration. The capsid protects the genome throughout this passage and mediates interactions with crucial host factors. It can thus be considered to be the master organizer of the post entry replication phase (T. Müller et al., Ann Rev Virology 2022).
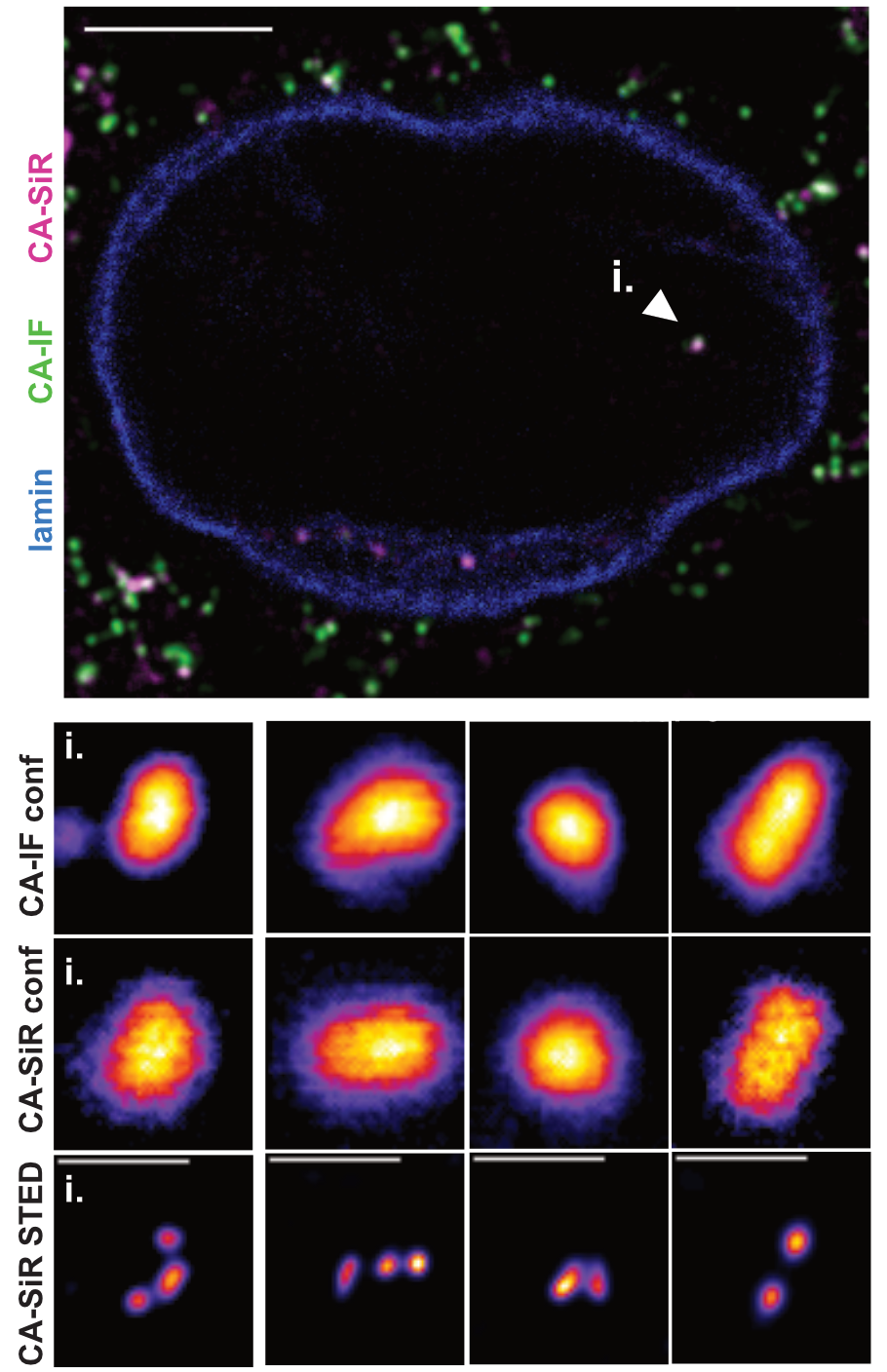
…..STED: Directly labeled HIV-1 CA inside the nucleus of
…………… TZM-bl cells.imaged by STED nanoscopy.
In the current funding period, we are working towards the following goals:
- In collaboration with the Fackler group, we will complete our ongoing studies on HIV-1 cell-cell transmission using a stable replication competent HIV-1 derivative labelled in Gag.
- Together with Kräusslich, we support the Briggs group in unravelling the role of the HIV-1 matrix protein in the early replication phase
- A main focus will be the fate of the HIV-1 capsid at and beyond the nuclear pore. We would like to find out whether capsids enter the nuceus through specialized nuclear pores, how capsids reach the integration site and what initiates genome uncoating. Within a closely interacting network with Kräusslich and Beck/Lusic together with Briggs and Fackler, we address these questions by studying the dynamics of nuclear events and the role of relevant host cell proteins.
- Our click-labeling strategy also allows us to detect the capsid by super-resolution microscopy. We want to exploit this to visualize interactions of HIV-1 capsids with the nuclear pore complex by the recently developed MINFLUX nanoscopy (collaboration with Kräusslich and Timo Zimmermann, EMBL Heidelberg).
- In contrast to HIV-1, moloney leukemia virus MLV is unable to enter an intact nucleus. It needs to wait for cell division to access the host cell genome. The capsids of the two related viruses thus both have crucial, but distinct functions in post-entry replication. We would like to understand key similarities and differences between these functions, and how this may be related to the different capsid morphologies. For this, we will adapt and apply the tools we developed for HIV-1 to MLV in collaboration with Kräusslich and perform comparative analyses.
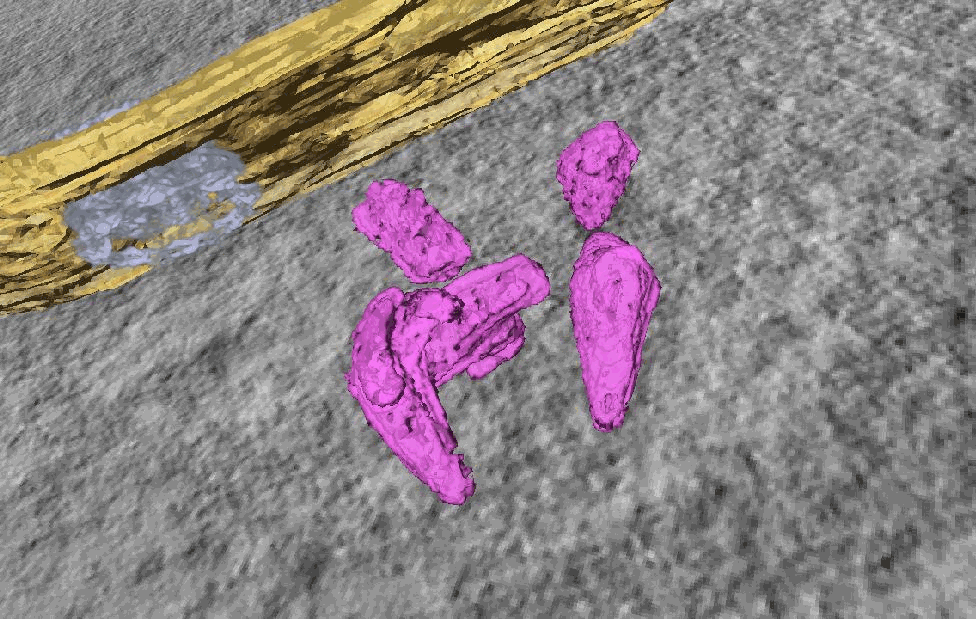
.
CLEM-ET analysis: Segmented and isosurface rendered structure of largely complete HIV-1 capsids in the nucleus of SupT1 cells.

HIV-1 cell-cell transmission through virological synapses
.
Project staff
Stephanie Ullrich
PhD student


