HIV-1 spread in complex cell systems

Bildnachweis: Universität Heidelberg, Kommunikation und Marketing
Project description – Summary – 3rd funding period
The goal of this project is to study aspects of HIV spread and pathogenesis that are not accessible to experimental investigation and parameter manipulation in classical cell culture models. To this end, we develop and apply new ex vivo and in vivo experimental models. During the first two funding periods, we established and characterized (a) ex vivo CD4 T lymphocyte cultures in synthetic 3D collagen matrices that allow long-term quantitative assessment of parameters governing HIV spread and (b) an adoptive transfer mouse model in conjunction with organotypic ex vivo cultures of human tonsil tissue for investigations of the role of HIV-1 on the humoral immune response to HIV infection. The infected 3D collagen cultures revealed that the extracellular environment shapes the mode and efficacy of HIV spread by limiting infection with cell-free virus particles while promoting motility-based, cell-associated virus transfer. In collaboration with Graw, Hamprecht, Rohr and Schwarz, two mathematical models were generated that describe the dynamics of virus replication and cell motility/virus transfer in these cultures and predictions about the key parameters governing the efficacy of HIV-1 spread in 3D collagen cultures were confirmed experimentally. Current work addresses the molecular mechanisms of the restriction to cell-free infection and the ultrastructural basis for enhanced cell-associated HIV-1 transmission in 3D collagen at cell contacts between donor and target CD4 T cells (collaboration Funaya/Schwab, Kräusslich, Müller). Work in the adoptive transfer mouse model and human tonsil cultures established that the HIV-1 pathogenesis factor Nef potently suppresses the helper function of CD4 T cells to B cells and thus the production of antibodies targeting infected cells and virus particles. In the current funding period we will build on these developments and fully integrate DCs as additional layer of complexity in our analyses. Two aims will be addressed to gain further new insight into HIV pathogenesis.
Aim 1 will seek to determine how the integration of dendritic cells (DCs) to the 3D culture system will affect the complex physical and functional interactions between HIV-1 and its target cells. In aim 2 we will adapt the ex vivo tonsil cultures and adoptive transfer mouse models to enable assessment of how HIV-1 infection of CD4 T cells affects their helper function directly to CD8 T cells and indirectly via cross-priming by DCs. Together, these analyses will yield insight into the mechanisms of HIV-1 spread and immune evasion in physiologically relevant complex cell culture and animal models.
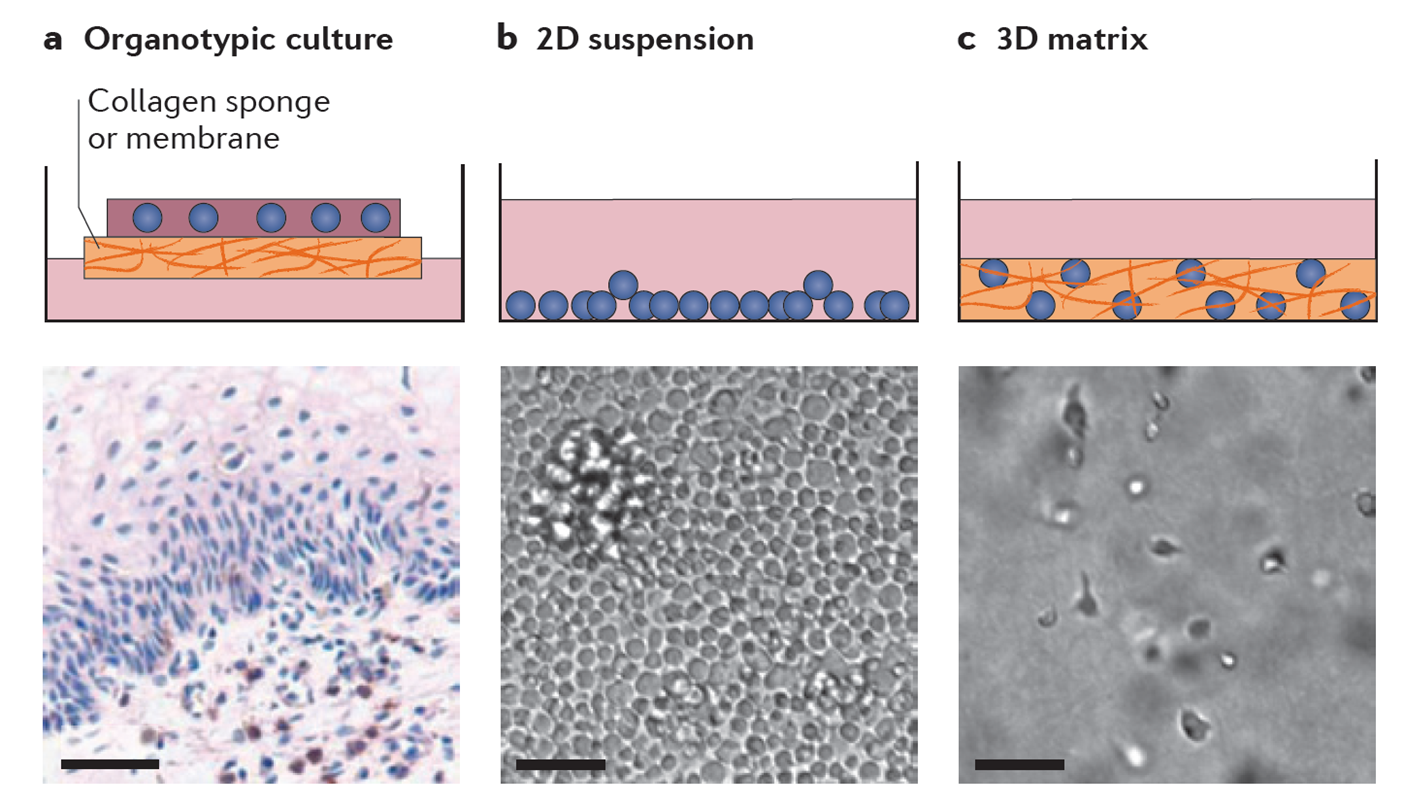
Ex vivo model systems (Reference: Fackler, O.T., Murooka, T.T., Imle, A. and Mempel, T.R. (2014). Adding new dimensions: Towards an integrative understanding of HIV-1 spread. Nat. Rev. Microbiol., 12: 563-574)

Schematic model of the impact of the 3D environment on HIV-1 spread. While the infectivity of cell-free HIV-1 particles is impaired in 3D collagen relative to suspension cultures, cell-cell trans-mission is promoted by the induction of longer lasting and closer cell-cell contacts (Sid Ahmed et al., Cells, 2020). The reduction of virion infectivity may e.g. result from impaired fusogenicity of HIV-1 Env after physical interaction with collagen fibers
.
Project staff

Samy Sid Ahmed
Postdoc
